- ››More information on molar mass and molecular weight. In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all.
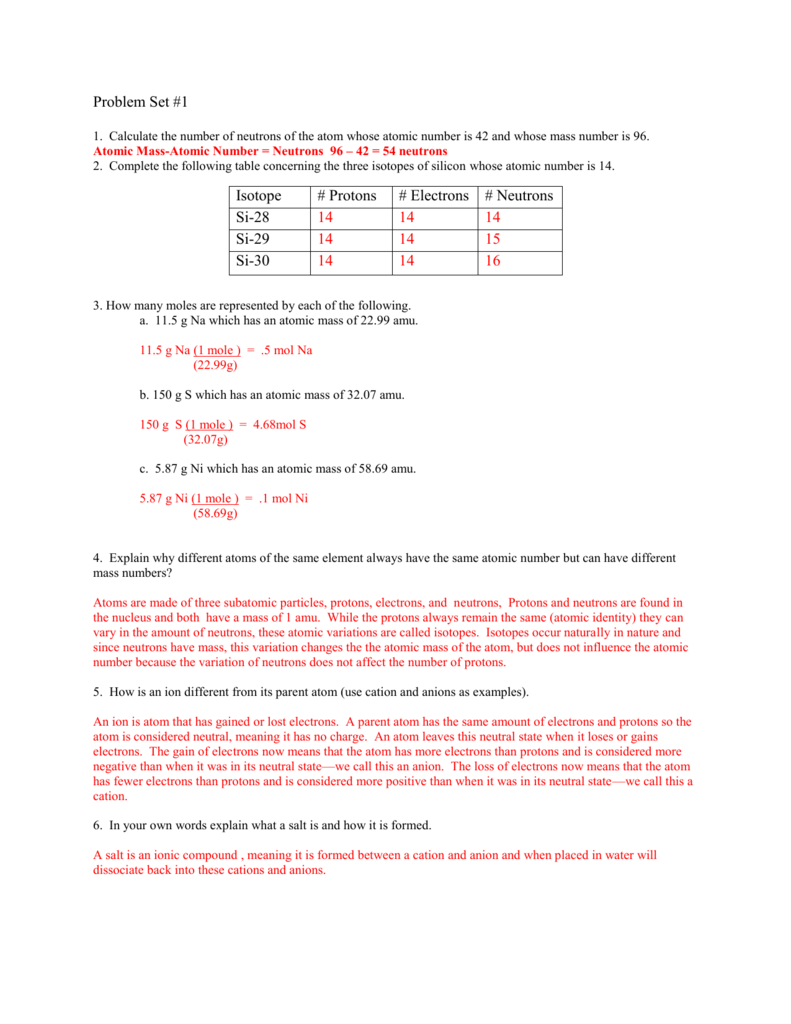
- NA World Services PO Box 9999 Van Nuys, California USA 91409 Telephone +1.818.773.9999 Fax +1.818.700.0700: Service Offices and Distribution Centers: WSO—Chatsworth.
- Na I Ground State 1s 2 2s 2 2p 6 3s 2 S 1 / 2 Ionization energy 41449.451 cm-1 (5.139076 eV) Ref. BBLB98 Na II Ground State 1s 2 2s 2 2p 6 1 S 0 Ionization energy 381390.2 cm-1 (47.2864 eV) Ref.

Has an atomic number double that of sodium’s. Has the lowest atomic number of any group 15 element. Is in group 1; has a higher atomic number than Cl but a lower atomic number than Br. Has between 35 and 55 protons and is in group 17. Has the lowest atomic mass on the periodic table. Group 2 element; has fewer protons than bromide, but more. Sodium is present in fair abundance in the sun and stars. The D lines of sodium are among the most prominent in the solar spectrum. Sodium is the fourth most abundant element on earth, comprising about 2.6% of the earth's crust; it is the most abundant of the alkali group of metals.
Element Sodium - Na
Comprehensive data on the chemical element Sodium is provided on this page; including scores of properties, element names in many languages, most known nuclides of Sodium. Common chemical compounds are also provided for many elements. In addition technical terms are linked to their definitions and the menu contains links to related articles that are a great aid in one's studies.
Sodium Menu
- Sodium Page One
- Sodium Page Two
- Sodium Page Three
Overview of Sodium
- Atomic Number: 11
- Group: 1
- Period: 3
- Series: Alkali Metals
Sodium's Name in Other Languages
- Latin: Natrium
- Czech: Sodík
- Croatian: Natrij
- French: Sodium
- German: Natrium - r
- Italian: Sodio
- Norwegian: Natrium
- Portuguese: Sódio
- Russian: Натрий
- Spanish: Sodio
- Swedish: Natrium

Atomic Structure of Sodium
- Atomic Radius: 2.23Å
- Atomic Volume: 23.7cm3/mol
- Covalent Radius: 1.54Å
- Cross Section (Thermal Neutron Capture) σa/barns: 0.53
- Crystal Structure: Cubic body centered
- Electron Configuration:
- 1s2 2s2p6 3s1
- Electrons per Energy Level: 2,8,1
- Shell Model
- Shell Model
- Ionic Radius: 1.02Å
- Filling Orbital: 3s1
- Number of Electrons (with no charge): 11
- Number of Neutrons (most common/stable nuclide): 12
- Number of Protons: 11
- Oxidation States: 1
- Valence Electrons: 3s1
- Electron Dot Model
- Electron Dot Model
Chemical Properties of Sodium
- Electrochemical Equivalent: 0.85775g/amp-hr
- Electron Work Function: 2.75eV
- Electronegativity: 0.93 (Pauling); 1.01 (Allrod Rochow)
- Heat of Fusion: 2.598kJ/mol
- Incompatibilities:
- Ionization Potential
- First: 5.139
- Second: 47.286
- Third: 71.641
- Valence Electron Potential (-eV): 14.1
Physical Properties of Sodium
- Atomic Mass Average: 22.98977
- Boiling Point: 1156K 883°C 1621°F
- Coefficient of lineal thermal expansion/K-1: 70.6E-6
- Conductivity
- Electrical: 0.21 106/cm Ω
Thermal: 1.41 W/cmK
- Electrical: 0.21 106/cm Ω
- Density: 0.971g/cc @ 300K
- Description:
- Soft silvery white alkali metal that reacts violently with water and oxidises rapidly when cut. Pure sodium must be stored in oil.
- Elastic Modulus:
- Bulk: 6.3/GPa
- Rigidity: 2.53/GPa
- Youngs: 6.8/GPa
- Enthalpy of Atomization: 108.4 kJ/mole @ 25°C
- Enthalpy of Fusion: 2.59 kJ/mole
- Enthalpy of Vaporization: 89.04 kJ/mole
- Flammablity Class:
- Freezing Point:see melting point
- Hardness Scale
- Brinell: 0.69 MN m-2
- Mohs: 0.5
- Heat of Vaporization: 96.96kJ/mol
- Melting Point: 371K 98°C 208°F
- Molar Volume: 23.7 cm3/mole
- Physical State (at 20°C & 1atm): Solid
- Specific Heat: 1.23J/gK
- Vapor Pressure = 0.0000143Pa@961°C
Na-24 Mass Number
Regulatory / Health
- CAS Number
- 7440-23-5
- OSHAPermissible Exposure Limit (PEL)
- No limits set by OSHA
- OSHA PEL Vacated 1989
- No limits set by OSHA
- NIOSHRecommended Exposure Limit (REL)
- No limits set by NIOSH
- Levels In Humans:
Note: this data represents naturally occuring levels of elements in the typical human, it DOES NOT represent recommended daily allowances.- Blood/mg dm-3: 1970
- Bone/p.p.m: 10000
- Liver/p.p.m: 2000-4000
- Muscle/p.p.m: 2600-7800
- Daily Dietary Intake: 2-15 g
- Total Mass In Avg. 70kg human: 100 g
- Discovery Year: 1807
- Name Origin:
- From soda (Na2CO3); Na from Latin natrium.
- Abundance of Sodium:
- Earth's Crust/p.p.m.: 23000
- Seawater/p.p.m.: 10500
- Atmosphere/p.p.m.: N/A
- Sun (Relative to H=1E12): 1910000
- Sources of Sodium:
- Obtained by electrolysis of melted sodium chloride (salt), borax and cryolite. Approximate annual world wide production: sodium metal, 200,000 tons; salt, 168,000,000 tons; sodium carbonate, 29,000,000 tons. Primary mining areas are Germany, Poland, Kenya USA.
- Uses of Sodium:
- Used in medicine, agriculture and photography. Liquid sodium is sometimes used to cool nuclear reactors. Also used in street lights, batteries, table salt (NaCl), and glass.
- Additional Notes:
- Sodium comes from the English word 'soda' and from mideval Latin sodanum which means headache remedy. Sodium is the sixth most abundant element on earth comprising 2.6% of the earth's crust. It is the most abundant of the alkali metals. It never exists in nature, but is prepared by electrolysis of absolutely dry fused sodium chloride. Sodium chloride is common table salt which is important in animal nutrition. Other important forms of sodium are soda ash(Na2CO3), baking soda (NaHCO3), Chili saltpeter (NaNO3) which is sodium nitrate. in nature sodium is fount in soda niter, cryolite, amphibole and zeolite.
Sodium Menu
- Sodium Page One
- Sodium Page Two
- Sodium Page Three
References
A list of reference sources used to compile the data provided on our periodic table of elements can be found on the main periodic table page.
Related Resources
- Anatomy of the Atom
Answers many questions regarding the structure of atoms. - Molarity, Molality and Normality
Introduces stoichiometry and explains the differences between molarity, molality and normality. - Molar Mass Calculations and Javascript Calculator
Molar mass calculations are explained and there is a JavaScript calculator to aid calculations. - Chemical Database
This database focuses on the most common chemical compounds used in the home and industry.
Citing this page
If you need to cite this page, you can copy this text:
Kenneth Barbalace. Periodic Table of Elements - Sodium - Na. EnvironmentalChemistry.com. 1995 - 2021. Accessed on-line: 4/25/2021
https://EnvironmentalChemistry.com/yogi/periodic/Na.html/
.
Linking to this page
If you would like to link to this page from your website, blog, etc., copy and paste this link code (in red) and modify it to suit your needs:
<a href='https://EnvironmentalChemistry.com/yogi/periodic/Na.html/'>echo Periodic Table of Elements: Sodium - Na (EnvironmentalChemistry.com)</a>- Comprehensive information for the element Sodium - Na is provided by this page including scores of properties, element names in many languages, most known nuclides and technical terms are linked to their definitions.
.
NOTICE: While linking to articles is encouraged, OUR ARTICLES MAY NOT BE COPIED TO OR REPUBLISHED ON ANOTHER WEBSITE UNDER ANY CIRCUMSTANCES.
PLEASE, if you like an article we published simply link to it on our website do not republish it.
Sodium Neutron
Molar mass of NaBr = 102.89377 g/mol
This compound is also known as Sodium Bromide.
Convert grams NaBr to moles or moles NaBr to grams
Molecular weight calculation:
22.98977 + 79.904
| Symbol | # of Atoms | Sodium | Na | 22.989770 | 1 | 22.343% | |
| Bromine | Br | 79.904 | 1 | 77.657% |

In chemistry, the formula weight is a quantity computed by multiplying the atomic weight (in atomic mass units) of each element in a chemical formula by the number of atoms of that element present in the formula, then adding all of these products together.
If the formula used in calculating molar mass is the molecular formula, the formula weight computed is the molecular weight. The percentage by weight of any atom or group of atoms in a compound can be computed by dividing the total weight of the atom (or group of atoms) in the formula by the formula weight and multiplying by 100.
Formula weights are especially useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are sometimes called equation weights.
The atomic weights used on this site come from NIST, the National Institute of Standards and Technology. We use the most common isotopes. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which may also be called standard atomic weight or average atomic mass.
Finding molar mass starts with units of grams per mole (g/mol). When calculating molecular weight of a chemical compound, it tells us how many grams are in one mole of that substance. The formula weight is simply the weight in atomic mass units of all the atoms in a given formula.
A common request on this site is to convert grams to moles. To complete this calculation, you have to know what substance you are trying to convert. The reason is that the molar mass of the substance affects the conversion. This site explains how to find molar mass.
Using the chemical formula of the compound and the periodic table of elements, we can add up the atomic weights and calculate molecular weight of the substance.
